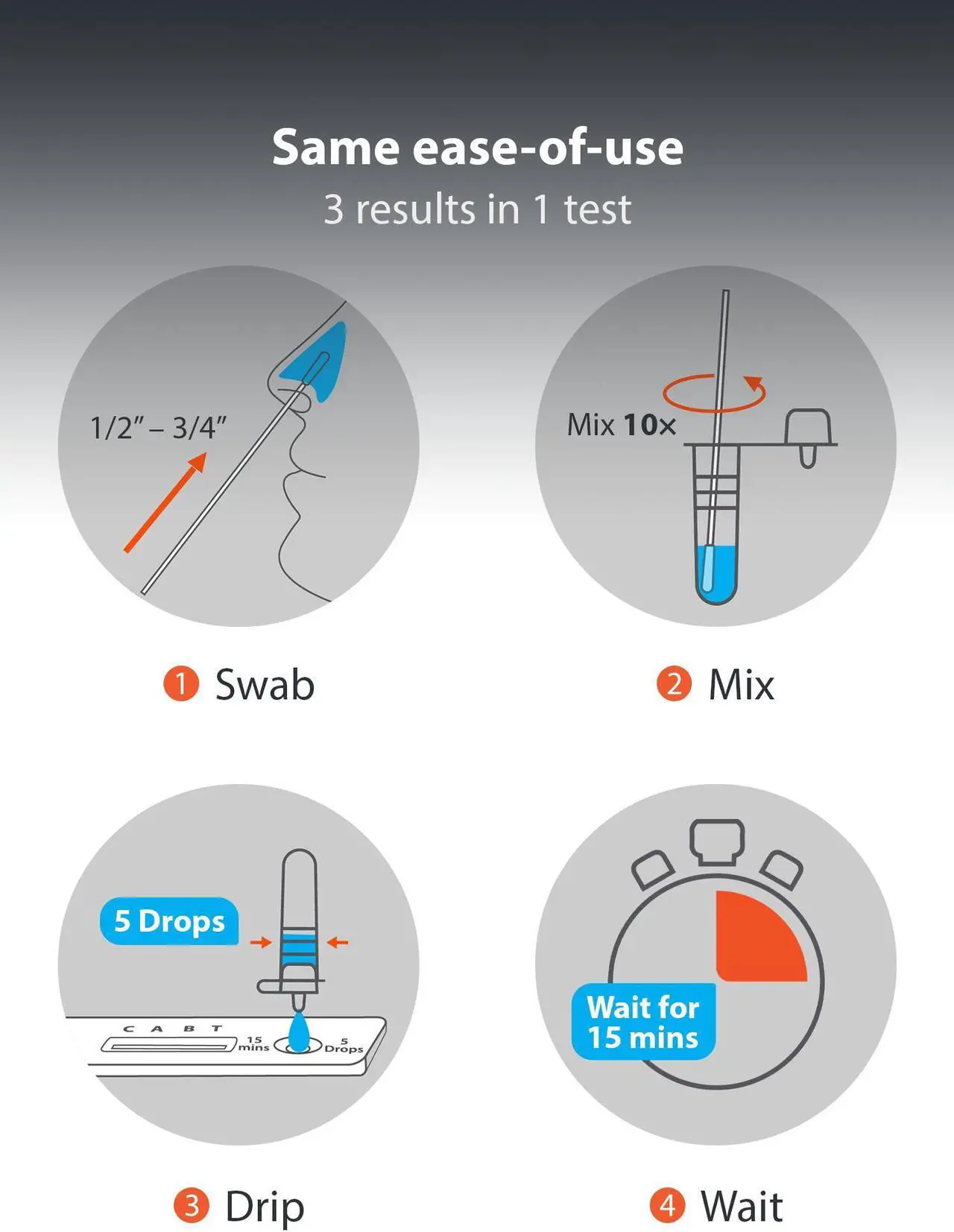
The iHealth COVID-19/Flu A&B Rapid Test is intended for over-the-counter (OTC) use and the qualitative detection of SARS-CoV-2, Influenza A and/or Influenza B nucleocapsid protein antigen in anterior nasal (nares) swab samples. This test does not determine if you had COVID-19/Flu A&B in the past or if you have immunity. The iHealth COVID-19/FLU A&B Rapid Test is a lateral flow immunoassay intended for the qualitative detection and differentiation of SARS-CoV-2, influenza A, and influenza B protein antigens. This test is authorized for non-prescription home use with self-collected anterior nares nasal swab specimens from individuals aged 14 years or older, or with adult-collected anterior nasal swab specimens from individuals two (2) years or older.
This test is only authorized for individuals with signs and symptoms of respiratory infection consistent with COVID-19 within the first for (4) days of symptom onset when tested at least twice over three days with at least 48 hours between tests.
The iHealth COVID-19/FLU A&B Rapid Test is only for use under the Food and Drug Administration's Emergency Use Authorization.